Graphite Density – Complete Guide, Properties & Applications
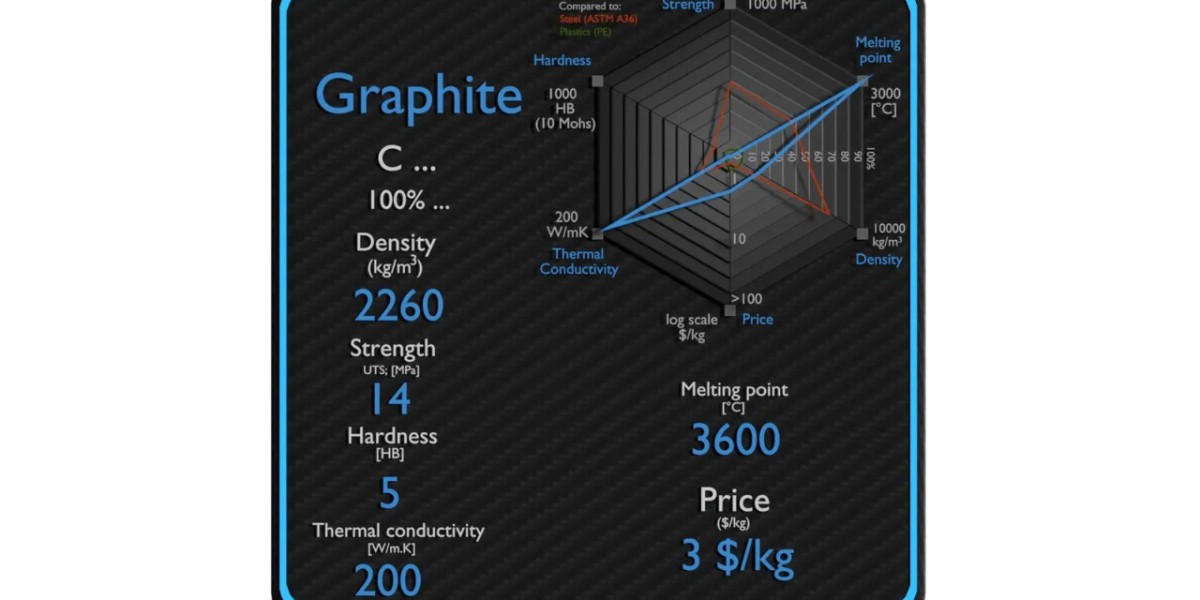
Graphite is one of the most versatile and widely used forms of carbon in modern industries. Its unique structure and properties make it suitable for applications ranging from batteries and lubricants to high-temperature industrial processes. One of the most important characteristics of graphite is its density. Understanding graphite density helps engineers, scientists, and manufacturers choose the right material for the right application. This guide explains graphite density, its properties, typical values, and key industrial applications in simple, professional English.
What is Graphite Density?
Density is the mass of a substance per unit volume. The formula is:
Density=MassVolume\text{Density} = \frac{\text{Mass}}{\text{Volume}}
For graphite, density describes how tightly carbon atoms are packed within its layered structure. Graphite has a layered arrangement of carbon atoms, where strong bonds hold atoms together within a layer, and weak forces exist between layers. This structure affects its density and, consequently, its properties.
Typical graphite density ranges between 2.09 and 2.23 grams per cubic centimeter (g/cm³). The exact value depends on:
Type of graphite (natural or synthetic)
Purity and composition
Porosity (amount of empty space in the material)
Processing methods, including heat and pressure
Properties of Graphite Related to Density
Graphite’s density is closely linked to its unique physical and chemical properties:
1. Electrical Conductivity
Graphite conducts electricity because electrons can move freely between its layers. Higher-density graphite often provides better conductivity, making it suitable for electrodes and battery components.
2. Thermal Stability
Graphite can withstand very high temperatures without melting. High-density graphite is preferred for industrial furnaces and refractory applications due to its strength and heat resistance.
3. Lubrication
Graphite layers can slide over each other easily. Low-density, porous graphite spreads more effectively and acts as a natural lubricant in machinery and industrial equipment.
4. Mechanical Strength
Density affects graphite’s strength. High-density graphite is stronger and more durable, making it ideal for structural components.
5. Chemical Resistance
Graphite is chemically inert under most conditions. Higher density and purity improve its stability in aggressive chemical environments.
Factors Affecting Graphite Density
Graphite density varies depending on several factors:
Type of Graphite
Natural Graphite: Density ~2.2 g/cm³. Found in mineral deposits and used for general applications.
Synthetic Graphite: Density ~2.1–2.25 g/cm³. Manufactured under controlled conditions for precise industrial use.
Purity: Impurities like metals or ash can reduce or vary density.
Porosity: Graphite with pores is lighter and has lower density. Porous graphite is often used in lubrication and lightweight materials.
Processing Conditions: Heat, pressure, and compression during manufacturing can reduce voids and slightly increase density.
How Graphite Density is Measured
Accurate density measurement is crucial for industrial and scientific applications. Common methods include:
Archimedes’ Principle: Measures bulk density using liquid displacement.
X-ray Diffraction (XRD): Estimates density from the crystal structure.
Pycnometer Method: Measures density precisely for powdered or small graphite samples.
These methods ensure that graphite meets the required specifications for different applications.
Industrial Applications of Graphite Based on Density
Graphite density directly determines its suitability for industrial applications:
1. Electrodes
High-density graphite is used in electric arc furnaces and other electrical applications. Its combination of strength, thermal stability, and conductivity makes it ideal for industrial electrodes.
2. Lubricants
Low-density, porous graphite serves as a dry lubricant. It spreads easily, reduces friction, and protects machinery from wear and tear.
3. Batteries
Graphite anodes in lithium-ion batteries require controlled density for efficiency, energy storage, and durability. High-density graphite improves battery performance.
4. Nuclear Reactors
Dense graphite is used as a neutron moderator. Its strength, thermal stability, and density ensure safe operation in nuclear reactors.
5. Refractory Materials
High-density graphite is used in furnace linings and molten metal containers because it can withstand extreme heat and mechanical stress.
Advantages of Understanding Graphite Density
Understanding graphite density allows industries to:
Select the right graphite for a specific application
Improve performance and efficiency of products
Ensure long-term durability of components
Optimize costs by using the correct density material
Graphite’s combination of lightweight, strength, conductivity, heat resistance, and chemical stability makes it invaluable in modern engineering.
Conclusion
Graphite density is a fundamental property that determines its performance and suitability for various applications. Low-density graphite is ideal for lubrication and lightweight applications, while high-density graphite is critical for electrodes, batteries, and nuclear reactors.
By understanding graphite density and its properties, engineers and manufacturers can make informed decisions to maximize efficiency, durability, and performance. With its unique structure, versatility, and range of industrial applications, graphite continues to be a vital material in modern technology and industry.